When discussing subatomic particles, two commonly mentioned ones are the proton and the alpha particle. They both play essential roles in the field of physics and have specific characteristics that distinguish one from the other. In this article, we will delve into the properties, behavior, and applications of protons and alpha particles to provide a comprehensive overview and comparison of these fundamental particles.
Protons:
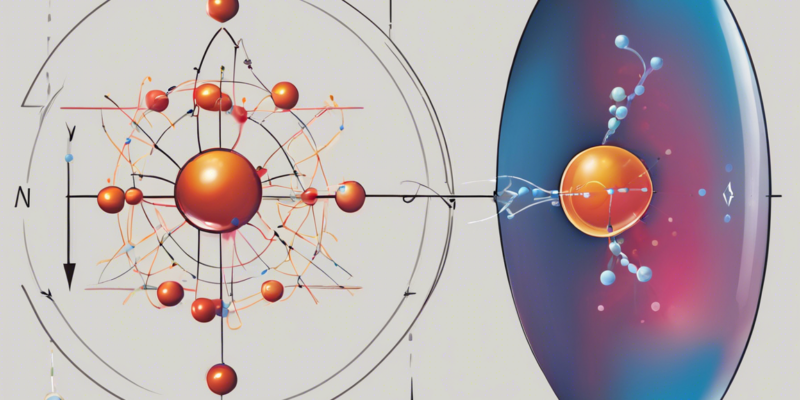
Protons are subatomic particles that are found in the nucleus of every atom. They have a positive electric charge, which is crucial for holding the nucleus together through electromagnetic force, along with neutrons. The number of protons in an atom determines its atomic number, which in turn defines the element. For example, an atom with one proton is hydrogen, while an atom with six protons is carbon.
Key Characteristics of Protons:
- Charge: Protons carry a positive charge of +1.
- Mass: The mass of a proton is approximately equal to that of a neutron.
- Location: Protons are located in the nucleus of an atom.
- Stability: Protons are considered stable particles and are not subject to radioactive decay.
- Symbol: In chemical equations and atomic structures, protons are represented by the symbol “p” or “p+”.
Alpha Particles:
Alpha particles, on the other hand, are made up of two protons and two neutrons bound together, essentially forming a helium-4 nucleus. They are commonly emitted during the process of radioactive decay, particularly in alpha decay, where a heavy nucleus sheds an alpha particle to increase stability.
Key Characteristics of Alpha Particles:
- Composition: Alpha particles consist of two protons and two neutrons.
- Charge: They carry a positive charge of +2, given the presence of two protons.
- Mass: Due to their composition, alpha particles have a larger mass compared to protons.
- Symbol: In equations and nuclear reactions, alpha particles are denoted by the symbol “α”.
- Penetrating Power: Alpha particles have low penetrating power and can be stopped by a piece of paper or human skin.
Comparison and Contrast:
Now, let’s compare protons and alpha particles in various aspects to better understand their differences and similarities:
1. Charge:
– Protons have a charge of +1, while alpha particles carry a charge of +2.
2. Mass:
– The mass of a proton is approximately 1 atomic mass unit (amu), whereas alpha particles have a mass of about 4 amu.
3. Composition:
– Protons are singular particles, while alpha particles consist of two protons and two neutrons bound together.
4. Application:
– Protons are integral in determining the chemical properties of elements, whereas alpha particles are commonly used in smoke detectors and in medical treatments for targeted radiation therapy.
5. Penetrating Power:
– Alpha particles have low penetrating power and can be blocked by a barrier, while protons have higher penetrating power and can penetrate deeper into matter.
6. Stability:
– Protons are stable particles found in the nucleus of atoms, while alpha particles are typically emitted from unstable, radioactive nuclei during decay processes.
Applications of Protons and Alpha Particles:
Both protons and alpha particles have unique applications and uses across various fields of science and technology. Some notable applications include:
Proton Applications:
1. Cancer Treatment: Proton therapy is used in cancer treatment to deliver targeted radiation to tumors, minimizing damage to surrounding healthy tissues.
2. Particle Accelerators: Protons are accelerated to high energies in particle accelerators for fundamental research in particle physics.
Alpha Particle Applications:
1. Smoke Detectors: Alpha particles are used in smoke detectors due to their ionizing properties, which help detect smoke particles.
2. Industrial Thickness Gauges: Alpha particles are utilized in industrial thickness gauges to measure the thickness of materials like paper and plastic.
Frequently Asked Questions (FAQs):
1. Are protons and alpha particles the same thing?
– No, protons are singular subatomic particles found in atomic nuclei, while alpha particles are composed of two protons and two neutrons.
2. Can alpha particles penetrate the human body?
– Alpha particles have low penetrating power and can be stopped by a barrier, such as a sheet of paper or skin, so they cannot penetrate the human body easily.
3. How are protons and alpha particles related to radiation therapy?
– Protons are used in proton therapy for cancer treatment, delivering targeted radiation, while alpha particles have been used in targeted radiation therapy for specific types of cancer.
4. Why are protons important in determining the identity of an element?
– The number of protons in an atom’s nucleus defines its atomic number, which determines the element’s identity, making protons crucial in differentiating elements.
5. Are alpha particles radioactive?
– Alpha particles are commonly emitted as a form of radiation from certain unstable nuclei during radioactive decay processes.
In conclusion, protons and alpha particles are fundamental components of atomic nuclei with distinct properties and applications. Understanding the unique characteristics and behaviors of these particles is essential in various scientific fields, ranging from nuclear physics to medical applications like cancer treatment. By comparing and contrasting protons and alpha particles, we gain insight into their roles in the microscopic world and their broader implications in science and technology.











Comments